This article is by Marco Colasanti extracted from Aquarium Chemistry: The Nitrogen Cycle: New Developments and New Prospects.
The nitrogen cycle plays a highly important role in a closed environment like that of an aquarium. Due to its presence, it is possible keep the fish and invertebrates alive, in a small viable space, therefore it is fundamental to learn to know it, mainly in respect of the life forms that we nurture.
Until a few years ago, it was thought that the nitrogen cycle in its complexity, was a complete linear process. However, most recent scientific discoveries have greatly revolutionized our well-established knowledge on the nitrogen cycle and on the micro-organisms involved in such processes. As a matter of fact, the global cycle of nitrogen in the environment, particularly in that of marine, has been integrated with at least three new links which include:
-the oxidation of ammonium by a particular group of micro-organisms, the archaeabacteria (AOA);
-the anaerobic reduction of nitrates into ammonium ion (DNRA);
-the anaerobic oxidation processes of ammonium (ANAMMOX).
In the first part of this article, I will try to review the essential and more predominant aspects of the nitrogen cycle: the transformation processes of the main components (atmospheric nitrogen, ammonium ion, nitrite, nitrate) and the role played by the bacterial species involved.
In the second part, new ways will be explored with particular reference on the role of bacteria, focusing on the implications that these new discoveries have brought in the global cycle of nitrogen.
The Canonical Nitrogen Cycle
Nitrogen (N) is an essential nutrient for all organisms, and it is a critical element of protein, vitamins and DNA, and is important in biochemical structures and process that define life.
Nitrogen exists in different states of oxidation and in many chemical forms and is quickly converted by the microorganisms both on earth and the sea.
In the marine environment, nitrogen is present in 5 forms:
–Gaseous nitrogen (N2), stable molecules that require specialized enzyme systems (present in some types of bacteria) for fixation and later use;
–Ammonium ion (NH4+), the most reduced natural specie of nitrogen, and the most biologically available in an oxygen-less environment;
–Nitrate ion (NO3-), the most oxidized form of nitrogen and mostly usable in an aerobic environment;
–Particulate organic nitrogen (PON), organic form of nitrogen predominant in sediments;
–Dissolved organic nitrogen (DON), a rich mixture of molecules with a wide range of composition.
A complex network of reactions links these nitrogen forms in processes that as a whole, is called the nitrogen cycle (Figure 1). The greatest source of nitrogen comes in the form of inert gas N2 (N ≡ N), representing 78% of the atmosphere. A small part of the atmospheric N2 is fixed by particular bacteria called nitrogen-fixing (nitrogen fixation) and is reduced to ammonium ion (NH4+) which can be easily usable for other organisms. In a marine environment which inhabited by particular bacteria, ammonium is quickly oxidized to nitrate in aerobic conditions (nitrification). Nitrate is then reduced again to an N2 gas in anaerobic conditions (denitrification), thereby completing the cycle (Figure 1).

Figure 1. Diagram of the marine nitrogen cycle.
Nitrogen Fixation and Ammonification
Nitrogen fixation
The biological fixation of nitrogen can be synthetically represented by the following global formula:
N2 + 8H+ + 6e- → 2NH4+
Which means that for each molecule of atmospheric nitrogen, 2 ammonium ions are formed with the absorption of 6 electrons and 6H+, this last process tends to increase the pH.
It is interesting to note that, ultimately, the ammonium ion in the water is in balance with ammonia (NH3) based on the following stoichiometry:
NH3 + H2O ↔ NH4+ + OH-
The concentration of the two chemical species relies largely on the pH, in short, the higher the alkalinity, the larger will be the quantity of ammonia; or proportion-wise, the lower the pH is (more acid), the larger will be the quantity of ammonium ion (less toxic than ammonia). As can be seen in Figure 2, in an average range of pH in the seawater, the percentage of NH4+ is higher (82-97%) compared to that of NH3 (3-18%).
As we have previously stated, the atmospheric nitrogen N2, before being incorporated into the biological molecules, has to be reduced to NH4+, through a series of reactions called biological fixation of nitrogen. Such reactions are catalyzed by a particular enzyme, nitrogenase, which is present in some nitrogen-fixing bacteria belonging mainly to Cyanobacteria phylum. One of the peculiar characteristics of this enzyme is that it comes irreversibly inhibited by the molecular oxygen (O2); and since fixation is a process that happens in an aerobic environment, it creates an apparent paradox. In reality, cyanobacteria are able to negotiate the activities of nitrogenase, an enzyme which is essentially anaerobic, with the inevitable presence of oxygen (resulting from photosynthetic processes), through not yet well-known mechanisms. In the marine environment, the nitrogen-fixing bacteria (some of which also belong to Clostridium and Azobacter genera) can be found both in free form and in symbiosis with other organisms (ex. Sponge).
But what is the source of nitrogen in an aquarium? Certainly, the biological fixation of nitrogen is an extremely important process in the ocean, but it has a limited role in the tank.
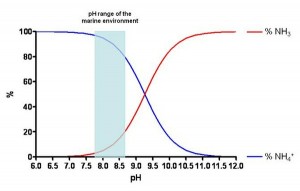
Figure 2. pH-dependent concentration of NH4+ and NH3 in the seawater
The main source of nitrogen is obtained from the nourishment both of the fish and invertebrates, particularly in the form of protein and single amino acids, assuming they are directly administered into the tank. Even minor vitamins and other molecules like the DNA, contain nitrogen but the quantity is decisively less than that of protein’s. In proteins, nitrogen forms a part of the framework and of some single amino-acids’ lateral chains, as the tryptophan, asparagin, glutamine, lysin, arginine, histidine.
The oxidative degradation of amino acids leads to the release of ammonia nitrogen into the tank. In what way? On one hand, the protein ingested by the fish or by the other organisms are broken down into single amino acids. In turn, amino acids can be used to build new proteins within the organism or be oxidized to supply energy. The degradation of amino acids by the animals leads to the elimination of varied by-products. For example, the fish release nitrogen as ammonia, while the majority of organisms may release it in the form of uric acid (fowls, reptiles), or urea (humans). On the other hand, in the presence of a strong organic charge, protein and amino acids in waste products, in sediments and in organic decay are decomposed in a process called ammonification, carried out by particular decomposer bacteria which release ammonium into the water by degrading the aminoacidic nitrogen.
Nitrification
Nitrification occurs in two distinct stages:
oxidation of ammonium to nitrite (nitrosation) and
oxidation of nitrite to nitrate (nitration).
1) Nitrosation: in the first stage, ammonium ion is oxidized to nitrite in two steps:
The first step is catalyzed by the enzyme, monooxygenase which forms the hydroxylamine by using O2 as oxidant:
2NH4+ + O2 → 2NH2OH + 2H+
In the second step, hydroxylamine is oxidized to nitrite by the enzyme hydroxylamine-dehydrogenase:
2NH2OH + 2O2 → 2H+ + 2H2O + 2NO2-
2) Nitration: the oxidation of nitrite to nitrate, which occurs through the activity of the nitrite oxidase enzyme, completes the process of nitrification:
2NO2- + O2 → 2NO3-
The conventional view of nitrification occurs in the presence of oxygen and anticipates the oxidation of ammonium to nitrate based on the following global synthetic formula (see Figure 1):
2NH4+ + 4O2 → 4H+ + 2H2O + 2NO3-
But who directs the music? The metabolic work of nitrification is entrusted to two groups of nitrifying bacteria:
bacteria which oxidize ammonium (ammonia-oxidizing bacteria or AOB), also called nitrous bacteria. They belong chiefly to the Nitrosococcus and Nitrosomonas species; bacteria which oxidize nitrite (Nitrite-oxidizing bacteria or NOB) also called nitric bacteria. They form a part of the Nitrobacter, Nitrococcus and Nitrospina species. The nitrifying bacteria are generally obliged aerobes and obliged chemoautorophs because they directly use CO2 as a source of carbon, while organic substances can be toxic.

